

Our Products
eye expert
Dômes Pharma is an international reference in veterinary ophthalmology with over 40 years of expertise in this field and distribution of its products in around thirty countries (Europe, USA, Australia, Asia).
Dômes Pharma is committed to providing veterinarians, technicians, and pet owners with:
- An extensive range of innovative ophthalmic products, from daily care and prevention to diagnostics and therapeutics
- Our teams’ scientific and technical expertise
- A broad range of services, including disease management guidelines and innovative educational experiences.

Launch in Canada
Dômes Pharma entered Canada in April 2025 with the launch of its range of corneal protectors for dogs, cats, horses and exotics:
REMEND™ 0.4
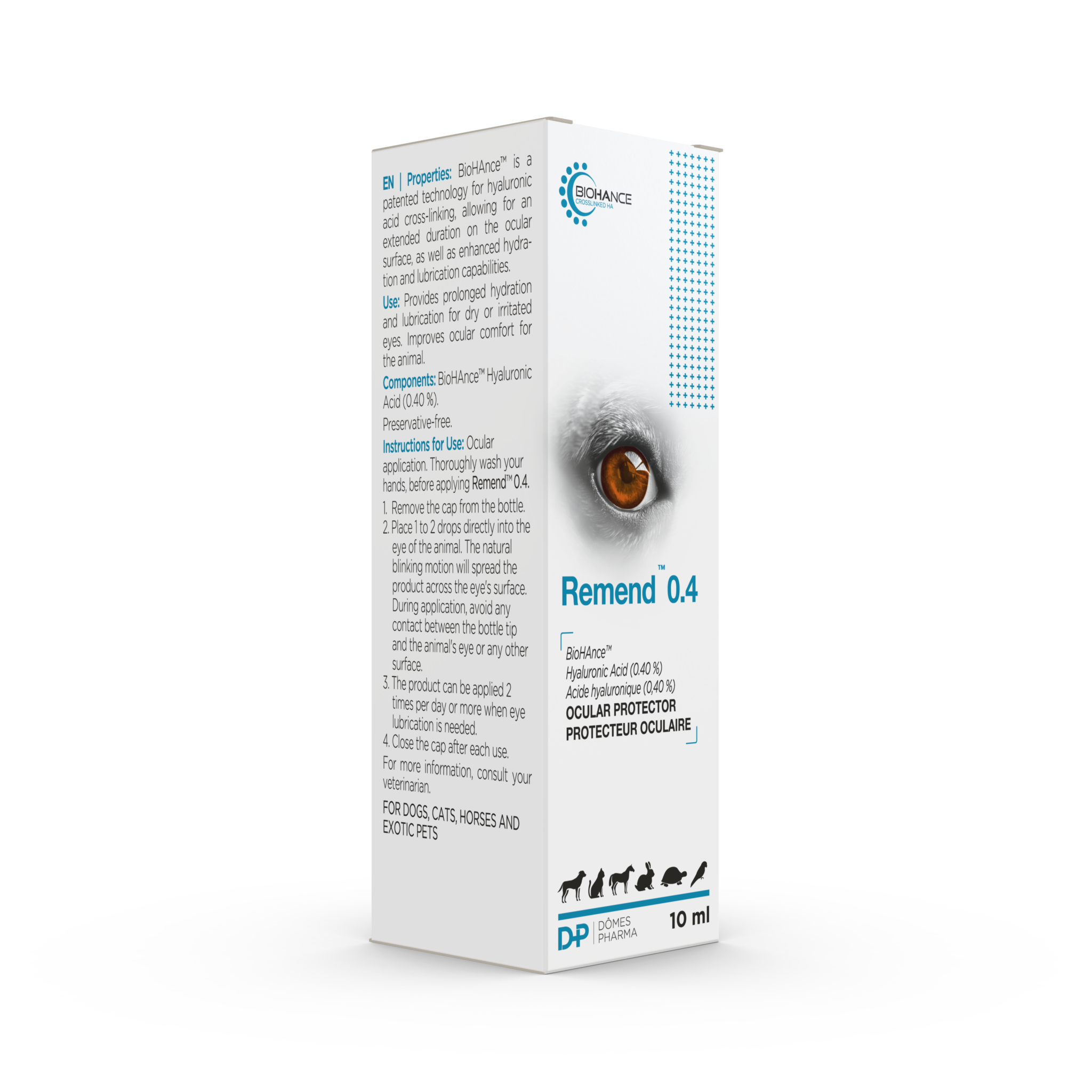
Ocular protector for dry or irritated eyes
REMEND™ 0.75

Support natural corneal regeneration
OCRY-GEL™
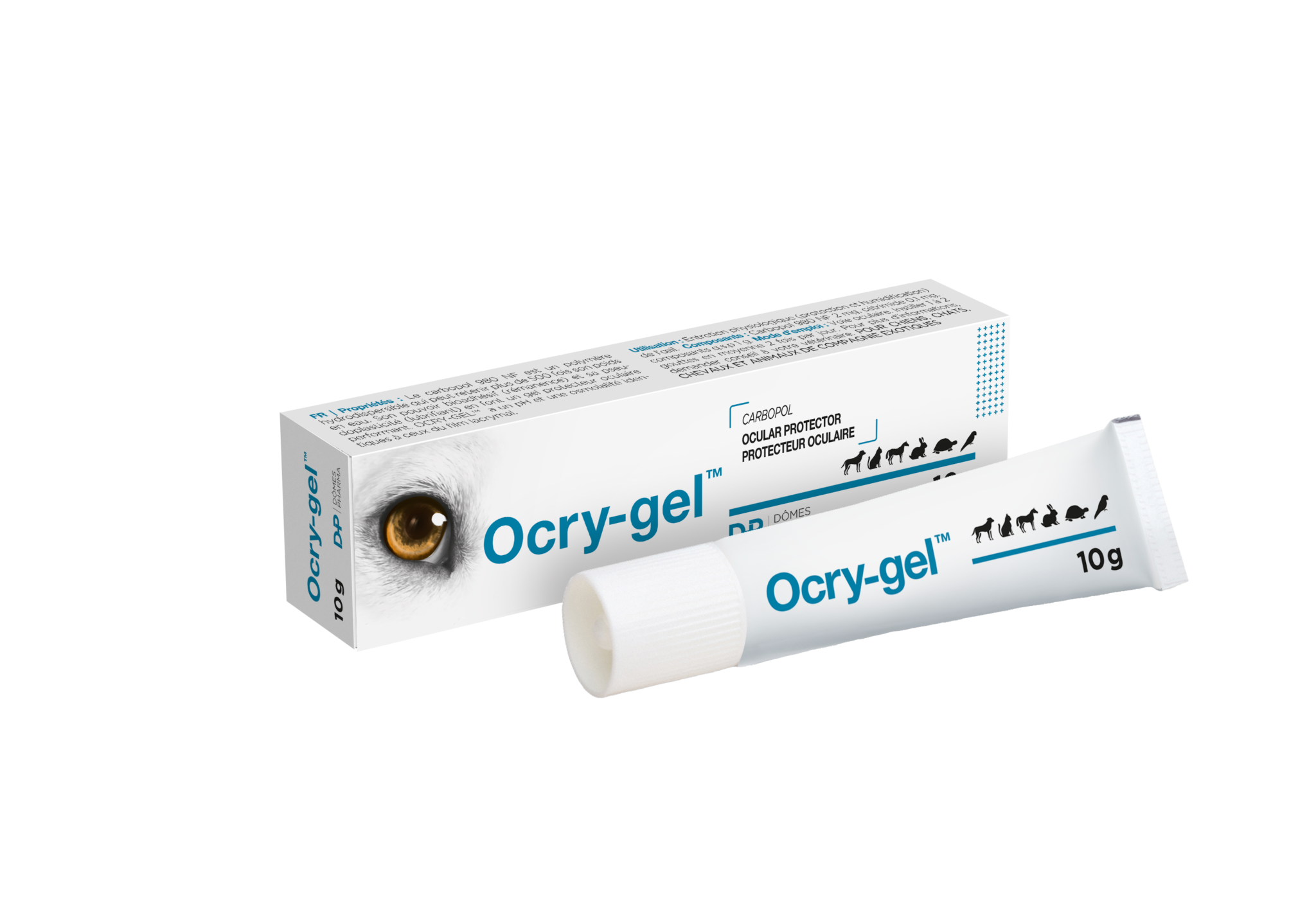
Ocular protector for situations of sedation, anesthesia or corneal exposure
REMEND™ products are manufactured using a patented hyaluronic acid cross-linking technology, BioHance™ technology.

BioHAnce™ is a patented technology which uses advanced bioengineering to create a molecular matrix of cross-linked hyaluronic acid. It produces a molecular scaffolding with unique physical and chemical properties, that enhances hydration, accelerates the body’s own healing processes and extends duration on ocular surface.
BioHAnce™ cross-linked hyaluronic acid has many benefits:
- Enhances hydration, lubrication, ocular comfort and helps stabilize the tear film1
- Increases residence time (vs linear hyaluronic acid)2,3
- Requires less applications, thus improving compliance
- Creates a thin barrier that soothes and protects the eye without altering vision
- 75% BioHAnce™ cross-linked hyaluronic acid supports faster corneal reconstruction (vs linear hyaluronic acid)4
- Prolongs the presence of topical treatments on the ocular surface5
- Does not bind to antibiotics (unlike autologous serum)6
- BioHAnce™ technology has been created specifically for animal health
- Very good tolerance:
- Preservative free
- Thiol cross-linking allows for a purified end product (no residual crosslinker which can be irritating or cause inflammation)
REMEND™ 0.4 – Lubricate and defend the cornea in case of dry or irritated eyes

10ml bottle
= provides around 30 days of use
- BioHAnce™ cross-linked HA: 0.4%
- No preservatives for a better tolerance
- Hydration, lubrication, eye comfort and helps stabilise the tear film1
- Increases residence time (vs linear hyaluronic acid): lasts 2-5 times longer than traditional artificial tears2,3
- Soothes and protects the eye without altering vision
- 1-2 drops per eye twice a day
REMEND™ 0.75 – Support natural corneal regeneration

3ml bottle
= provides around 15 days of use
- BioHAnce™ highly concentrated cross-linked HA: 0.75%
- No preservatives for a better tolerance
- 75% BioHAnce™ cross-linked HA supports faster corneal reconstruction (vs linear hyaluronic acid): up to 34%4
- Prolongs the presence of topical treatments on the ocular surface5
- 1-2 drops twice a day onto the affected eye until corneal reepithelialization is achieved
- Shelf life after opening = 1 month
OCRY-GEL™ – Provide lubrication and hydration during situations of sedation, anesthesia or corneal exposure

10g tube
- Carbomer-based gel: carbopol 980 NF, a water dispersible polymer that can retain over 500 times its weight in water
- Ergonomic nozzle
- pH and osmolarity the same as the tear film
- No white streaks upon drying
- 5-1 cm spread over the corneal surface on average twice a day or as necessary (every 20-30 minutes in anaesthesia situations)
Plummer CE, Martins BC, Bolch C, Martinez PS, Carbia BE (2022). Evaluation of topically applied cross-linked hyaluronic acid (Remend™) on the ocular surface of clinically healthy dogs. College of Veterinary Medicine, University of Florida; School of Veterinary Medicine, University of California- Davis; Institute for Vision Research, University of Florida. ACVO 2022.
Montiani-Ferreira F, Atzet SK, Fankhauser AD, Behan EK, Haeussler DJ (2022). Fluorometric evaluation of cross-linked vs linear hyaluronic acid eye lubricants. SentrX Animal Care; Veterinary Medicine Department, Federal University of Paraná; Animal Eye Institute. ACVO 2022.
Bedos L, Allbaugh RA, Roy MM, Kubai MA, Sebbag L. Precorneal retention time of ocular lubricants in dogs. Iowa State University College of Veterinary Medicine; Koret Schoolof Veterinary Medicine, The Hebrew University of Jerusalem.
Williams DL, Wirostko BM,Gum G, Mann BK (2017). Topical cross-linked HA-based hydrogel accelerates closure of corneal epithelial defects and repair of stromal ulceration in companion animals. Invest Ophthalmol Vis Sci. ; 58:4616–4622.
Sebbag L, Ortaeskinaz E, Goncharov Y, Ofri R, Arad D. (2023) Cross-linked hyaluronic acid enhances tear film concentrations of cefazolin sodium in canine eyes. Koret School of Veterinary Medicine, The Hebrew University of Jerusalem, Rehovot, Israel – ACVO Thursday, September 21st, 2023.
Atzet SK, Fankhauser AD, Behan EK, Mann, Brenda K (2022). Evaluation of crosslinked hyaluronic acid gel drops and therapeutic combinations for ophthalmic infections. Poster session. In : ACVO Conference 2022.